Aspartic Acid at Ph 3
4- Amino-pteroyl aspartic acid or its salts PDL. Most biochemistry courses will require you to know the following.
Prin prefixele di tri etc se arată numărul de grupe amino și carboxil iar poziția relativă a două grupe funcționale se precizează cu literele grecești α β γ δ ε în care acidul este α dacă grupările.
. Lide Handbook of Chemistry and Physics 72nd. Each molecule contains a central carbon C atom called the α-carbon to which both an amino and. She has taught science courses at the high school.
Asparaginsäure abgekürzt Asp oder D ist in ihrer natürlichen L-Form eine der proteinogenen α-Aminosäuren. The amino acid name the structure the pKa of ionizable hydrogens and both the 3-letter and 1-letter shorthand. Including but not limited to citric acid glycolic acid lactic acid malic acid mandelic acid ammonium glycolate.
Il farmacista francese Auguste-Arthur Plisson nel 1827 isolò lacido aspartico dopo aver fatto reagire lasparagina ottenuta dal succo di asparago con idrossido di piomboChiamò lacido ottenuto acide aspartique dal latino aspăragus asparago acido aspartico in italiano. 4 pl is the pH at the isoelectric point. GB 523 2392 69.
Wie die anderen Aminosäuren liegt Asparaginsäure im Körper normalerweise zwitterionisch vor. 00892796 Registered Charity no. The peptide net charge calculator at a given pH is based on the formula below.
All biological tissues contain amino acidsAll amino acids except glycine. Lysine and aspartic acid proline and glycine glutamine and arginine Correct Wrong. Amino acid any of a group of organic molecules that consist of a basic amino group NH2 an acidic carboxyl group COOH and an organic R group or side chain that is unique to each amino acid.
Helmenstine holds a PhD. In biomedical sciences and is a science writer educator and consultant. Always keep in mind structure gives function.
Portland Press Tel 44 020 3880 2795. Molecular weight of the amino acid residues. Sharp biting or sour in manner disposition or nature.
Sharply clear discerning or pointed. Adjective sour sharp or biting to the taste. The term amino acid is short for α-amino alpha-amino carboxylic acid.
In most acid stable proteins such as pepsin and the soxF protein from Sulfolobus acidocaldarius there is an overabundance of acidic residues which minimizes low pH destabilization induced by a buildup of positive charge. Studies of proteins adapted to low pH have revealed a few general mechanisms by which proteins can achieve acid stability. Formulations containing alpha hydroxy acids alone or in combination at concentrations of greater than 30 andor with a pH lower than 30.
Zusätzlich ist jedoch die zweite Carboxygruppe deprotoniert darum wird in der Biochemie statt L-Asparaginsäure oft die Bezeichnung L-Aspartat verwendet. Amino acid dating is a dating technique used to estimate the age of a specimen in paleobiology molecular paleontology archaeology forensic science taphonomy sedimentary geology and other fields. 2 pKb is the negative of the logarithm of the dissociation constant for the -NH3 group.
This technique relates changes in amino acid molecules to the time elapsed since they were formed. Aminoacizii se denumesc folosind cuvântul acid urmat de amino și numele acidului corespunzător. You Need to Review the Amino Acids.
Portland Press Registered address First Floor 10 Queen Street Place London EC4R 1BE Mailing address 1 Naoroji Street London WC1X 0GB. 1 pKa is the negative of the logarithm of the dissociation constant for the -COOH group. For the molecular weight amino acid calculator you can enter the 1- or 3- letter code of the desired amino acid and the tool will provide the value the same way it would calculate peptide molecular weight.
Errors in amino acid placement do occur and can lead to cell death in some instances. Biochemical Society Tel 44 020 3880 2793. Ritthausen nel 1868 isolò lacido aspartico da una proteina idrolizzata.
3 pKx is the negative of the logarithm of the dissociation constant for any other group in the molecule.
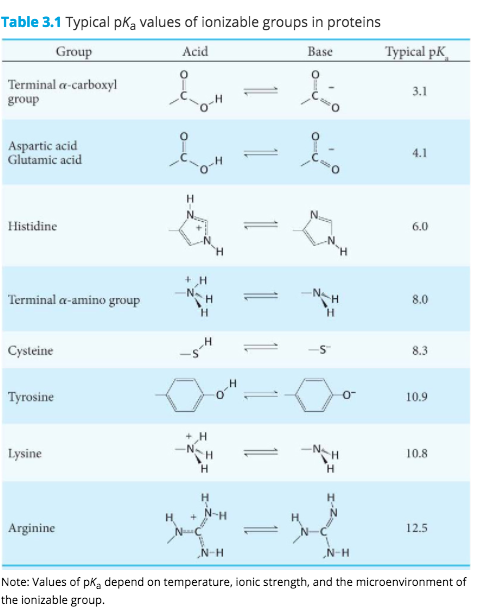
Solved 3 For Aspartic Acid At Ph 7 What Is The Ratio Of Chegg Com
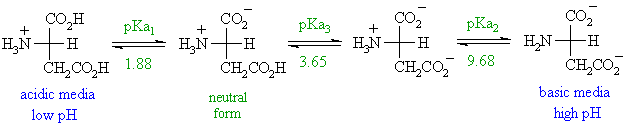
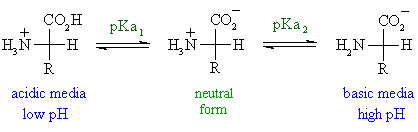
No comments for "Aspartic Acid at Ph 3"
Post a Comment